Solid calcium carbonate decomposes into carbon dioxide gas and solid calcium oxide. Calcium oxide CaO CID 14778 - structure chemical names physical and chemical properties classification patents literature biological activities safety.

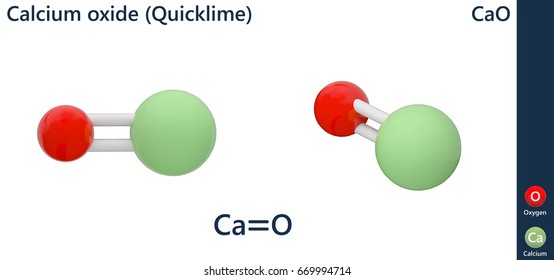
Calcium Oxide Lime Preparation Structure Properties Uses
Lets start by looking at the arrangement of particles in solids.

. The solid wastes may be biodegradable or non-biodegradable. Avoid contact with solid. There are three solids - the metal spoon the glass and the wooden table.
Is calcium oxide a liquid a solid or a gas. When heated solid copperII carbonate decomposes to solid copperII oxide and carbon dioxide gas. Solid wastes include solid portions of the discarded material such as glass bottles crockeries plastic containers metals and radioactive wastes.
Notify local health and pollution control agencies. Calcium oxide is an ionic compound. There is liquid water.
Predict its physical state solid liquid or gas at room temperature. All of the solids in the photo have a fixed shape. Ca OH2 is a solid which is sparingly soluble in water.
When 110 g of calcium metal is reacted with water 500 g of calcium hydroxide is produced. A How many moles of CaO can form from the. Solid calcium carbonate goes by the chemical formula.
For example calcium reacts. The balanced equation in a reaction involving the decomposition of calcium carbonate into solid calcium oxide and carbon dioxide gas would be. Its traditionally known as slaked lime.
Many metals react with oxygen gas to form the metal oxide. V Volume of the gas 5L R Universal gas. A small sample of CaCO3 is heated and the carbon dioxide that evolves is collected in a 125-mL flask.
The management of waste is another way of conservation of resources. And of course there is air all around it - a mixture of gases. Furthermore when matter approaches absolute zero it tends to enter into a different state where macroscopic particles take on.
Yes Oxygen is a non-metal in Group 6. Calcium Oxide is a solid. Write a balanced chemical equation for this reaction.
At absolute zero there is no kinetic energy. Is Ca Oh 2 a solid liquid or gas. -2 Is citric acid a weak or strong acid.
Solid calcium oxide and carbon dioxide gas combine to produce solid calcium carbonate. CALCIUM OXIDE CAO CAUTIONARY RESPONSE INFORMATION Common Synonyms Solid granules White to grey Odorless Sinks and reacts violently with water. The reason why Ca OH2 does not dissolve well is due to its solubility product.
Management of Solid Waste. Calcium chloride is an excellent desiccant which as it passes from a solid to a liquid state can absorb more than 35 times its weight in water. Even in its liquid state as a.
Solid calcium oxide is exposed to a stream of carbon dioxide gas. Quicklime Unslaked lime Keep people away. Ing unexpected changes in the gas composition gas flow or ambient conditions.
Balance equation for calcium metal. Solid Calcium Oxide And Gaseous Water Are Produced By Chegg Com 5 1 Writing And Balancing Chemical Equations Problems Chemistry Libretexts Write Balanced Equation For The Reaction Of Calcium Chegg Com Writing And Balancing Chemical Equations From Words Unit 9 Chemical Equations And Reactions Balancing Notes L. Wear rubber overclothing including gloves.
Answer 1 of 5. Solid calcium oxide goes by the chemical formula. The Ideal-Gas Law Solution When solid calcium carbonate CaCO3is heated it decomposes into solid calcium oxide CaO and carbon dioxide gas CO2.
Indicate the phases using abbreviation s l or g for solid liquid or gas respectively. 2Cas O2g-- 2CaOs Calculate the mass of calcium prepared from 420g of Ca and 280g of O2. The gas collected in the flask has a pressure of 195 atm at a temperature of 45 C.
Water appears to boil. A key ingredient in their mortar was quicklime calcium oxide which they produced by roasting limestone calcium carbonate. Adding excess water to a small amount of Ca OH2 we can get an aqueous solution of Ca OH2 more commonly known as lime water.
Solid Is N the correct symbol for nitrogen. O GASES LIQUIDS AND SOLIDS DE Solving for a gaseous reactant Ancient Romans built often out of bricks and mortar. The almond shape also minimizes pressure drop which is critical in wellhead operations.
Write a balanced chemical equation for this reaction. This tells us that it will produce ions that have a charge of. Give the balanced chemical equation including phases that describes this reaction.
Thats why its so difficult to even approach absolute zero. Carbon dioxide gas goes by the formula. Show transcribed image text Expert Answer 100 1 rating Applying ideal gas equation PV nRT P nRT V Where P Pressure of the gas.

10 Uses Of Calcium Oxide In Daily Life Nanografi Nano Technology
0 Comments